Abstract
Introduction Patients with myelodysplastic syndromes (MDS) often have a poor quality of life (QoL) as a consequence of their disease and resulting cytopenias, transfusion and treatment needs, and high healthcare resource use. The time spent in healthcare facilities for typical patients with lower-risk (LR) MDS is not known and could provide a quantifiable measure of burden of disease, as each interaction with the healthcare system has the potential to cause lifestyle disruptions. Here, we describe the impact of different therapies on lifestyle disruptions in patients with LR-MDS from the CONNECT® Myeloid Disease Registry (NCT01688011).
Methods Patients in the LR-MDS cohort were ≥18 years old and newly diagnosed with Low or Intermediate-1 risk MDS according to the International Prognostic Scoring System (IPSS) in the 60 days before enrollment. Patients were classified by first line of therapy (first treatment received during the first 45 days after diagnosis) into groups: no treatment, erythropoiesis-stimulating agents (ESAs), parenteral hypomethylating agents (HMAs), immunomodulatory drugs (IMiDs), transfusions only, and other. Mean (standard deviation [SD]) days of disruption were calculated per month (mo) for each quarterly period during the 12 mo after diagnosis and accounted for all treatments and disruptions during that time period, including those after the first line of therapy. Events considered to be lifestyle-disruptive were transfusions, emergency room visits, hospitalizations, clinic/lab visits, start of therapy, and parenteral chemotherapy treatment cycles, that is, those necessitating a clinic or hospital visit. Each treatment event in a healthcare setting was counted as 1 day of disruption.
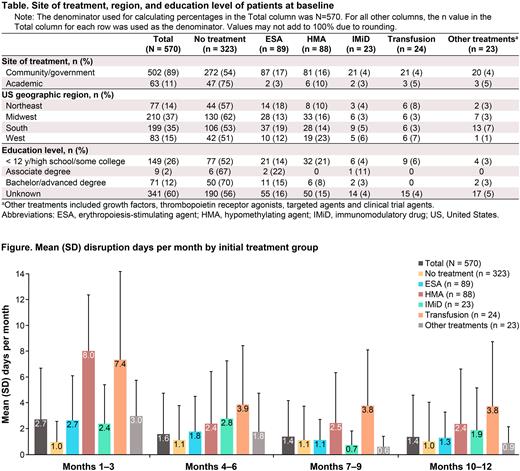
Results 570 patients with LR-MDS were enrolled as of February 18, 2022. Median age was 75 years, and most patients were male (67%), white (89%), had Eastern Cooperative Oncology Group scores of 0 or 1 (81%), and had mild frailty (82%). The proportion of patients receiving ESA and HMA appeared higher in the community/government vs academic sites (17% vs 3% and 16% vs 10%, respectively; Table). Overall, patients experienced a mean (SD) disruption of 2.73 (3.97) d/mo during the first 3 mo of treatment (Figure). Mean (SD) disruption decreased to 1.60 (3.15) d/mo during mo 4-6 and, thereafter, remained stable (1.39 [2.81] and 1.37 [3.23] d/mo during mo 7-9 and 10-12, respectively). Most patients in each treatment group experienced a similar pattern of higher disruption during the first 3 mo that decreased to a relatively stable level from mo 4-12. During the first 3 mo of treatment, lifestyle disruptions ranged from ~1 d/mo to ~8 d/mo depending on initial treatment. Patients receiving transfusions or HMAs as initial therapy experienced the most lifestyle disruptions. Mean (SD) disruptions during the first 3 mo were 7.35 (6.85) d/mo in the transfusion group and 8.04 (4.34) d/mo in the HMA group. Patients receiving IMiD agents as initial therapy experienced the fewest lifestyle disruptions; mean (SD) disruptions were 2.41 (3.00) d/mo during the first 3 mo and <2 d/mo from mo 7-12. Patients who received no treatment during their first 45 d after diagnosis experienced disruptions of ~1 d/mo in each period. Among patients in the HMA group who died (n=47), mean (SD) disruptions were 6.91 (6.45) d/mo during the final 3 mo of life; 1.47 (2.05) d/mo were associated with treatment, and 5.44 (5.64) d/mo were due to transfusions or other reasons requiring clinic/hospital visits. In contrast to a decrease in disruption in d/mo over time, mean (SD) EQ-5D scores remained stable throughout the treatment period (mo 1-3, 0.79 [0.18]; mo 4-6, 0.79 [0.18]; mo 7-9, 0.79 [0.18]; mo 10-12, 0.78 [0.18]).
Conclusions All treatment options were disruptive to patients’ lifestyles; patients who received transfusions or parenteral HMAs as their first treatment experienced the most disruptions. The impact was generally highest in the first 3 mo after diagnosis but continued at a lower level during the subsequent 4-12 mo. The transfusions group consistently experienced the most disruptions after the initial 3 mo. Current QoL measures, often used in clinical trials, do not account for meaningful lifestyle disruptions that could impact a patient's quality of life. These data suggest that some of the most common QoL instruments are not able to quantifiably measure and reflect burden of disease and treatment in patients with MDS.
Disclosures
Sekeres:Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; Bristol Myers-Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kurome: Membership on an entity's Board of Directors or advisory committees. Garcia-Manero:AbbVie: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Gilead Sciences: Research Funding; Curis: Honoraria, Research Funding; Aprea: Honoraria; Astex: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Grinblatt:Astellas Pharma: Consultancy, Research Funding; AstraZeneca: Consultancy; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy; ConcertAI: Other: Medical publication support. Komrokji:Acceleron Pharma: Consultancy; Servier: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; Geron: Consultancy; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Savona:Novartis: Consultancy; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding; Incyte Corporation: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Takeda: Consultancy; Astex Pharmaceuticals: Research Funding; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding; Geron: Consultancy; Sierra Oncology: Consultancy, Other: travel expenses; AbbVie: Consultancy, Other: travel expenses; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Taiho Pharmaceutical: Consultancy; Forma: Consultancy. Erba:ImmunoGen: Consultancy, Research Funding; Incyte: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; MacroGenics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; Covance (Abbvie): Consultancy, Other: Independent Review Committee, Research Funding; Janssen Oncology: Consultancy; Trillium Therapeutics: Consultancy; Takeda: Consultancy; Kura Oncology: Consultancy; Astellas Pharma: Consultancy; Glycomimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Celgene: Consultancy, Other, Speakers Bureau; Amgen: Consultancy, Research Funding; Agios: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Forma Therapeutics: Research Funding; Gilead/Forty Seven: Research Funding; PTC therapeutics: Research Funding; ALX Oncology: Research Funding; Pfizer: Consultancy. Roboz:CTI: Research Funding; Mofitt Cancer Center: Research Funding; Array BioPharma: Other: Travel and accommodation expenses; Astellas: Consultancy; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Karyopharm Therapeutics: Research Funding; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Amgen: Consultancy; Onconova Therapeutics: Research Funding; Jasper Therapeutics: Consultancy; Jazz: Consultancy, Other: travel; Actinium: Consultancy; Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Daiichi Sankyo: Consultancy; Agios: Other: travel, Research Funding; Amgen: Consultancy, Other: travel; Clovis Oncology: Other: Travel and accommodation expenses; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; Agios: Consultancy, Research Funding; Tensha Therapeutics: Research Funding; Takeda: Consultancy; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Mesoblast: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; Helsinn Therapeutics: Consultancy; Roche: Consultancy; Otsuka: Consultancy; MEI Pharma: Consultancy, Research Funding; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; Celltrion: Consultancy, Other: Travel and accommodation expenses; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Sandoz: Consultancy, Other: Travel and accommodation expenses; Bayer: Consultancy, Other: Travel and accommodation expenses; MedImmune: Consultancy, Research Funding. DeGutis:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kiselev:Juno: Current holder of stock options in a privately-held company; Celgene: Current holder of stock options in a privately-held company; Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Yu:BMS: Current Employment, Current equity holder in publicly-traded company, Other: travel and accommodation expenses. Makinde:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. McBride:Bristol Myers Squibb: Current Employment. Scott:Celgene: Consultancy, Honoraria, Other: Advisor Panel; Bristol Myers Squibb: Consultancy, Honoraria, Other: Advisory Panel, Research Funding; Alexion: Consultancy; Johnson and Johnson: Other: data and safety monitoring board; Incyte: Consultancy; Novartis: Other: Advisory Panel, Research Funding; Jazz Pharmaceuticals: Other: Advisory Panel; Nektar: Other: data and safety monitoring board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal